Manufacturing of veterinary drugs
Служба качества состоит из отдела обеспечения качества, отдела контроля качества и химико-аналитической лаборатории, оснащенной современным оборудованием для проведения всех необходимых испытаний.
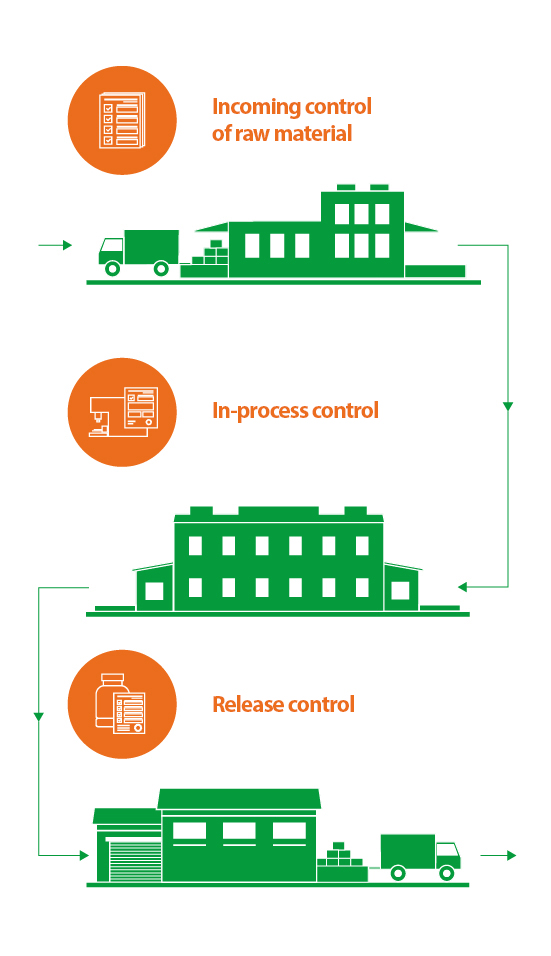
1. Incoming control of raw material
Each new purchased batch of raw material and feedstock undergoes laboratory tests stipulated by regulatory documentation and based on their findings an analytical sheet is drawn up. If the test is passed unremarkably, the raw material is transferred to the production facilities. Otherwise, it is returned to the supplier.
2. In-process control
Raw material and auxiliary materials that have successfully passed the incoming control are transferred to the manufacturing sites. The quality control department conducts inter-operation control at each process stage of drug production. Should the quality indicators of the product exceed the established limits, the technological process is suspended in order to adjust the quality of the product to the appropriate level.
3. Release control
The last step is the release control of the finished product. The success of this stage depends on the quality of each manufacturing operation and each test performed at the previous stages. Samples of the finished drugs, which have passed all the stages of technological process, are taken to conduct extended laboratory tests in order to confirm the quality of the produced batch. After a thorough study of the documents filled in during the manufacturing process and quality control of each drug batch, the Qualified Person certified by the federal authority issues a sales authorization or, if discrepancies are found, sends the products for revision.





- 1. Finished product warehouse
- 2. Raw material warehouse
- 3. Warehouses of packaging and auxiliary materials
- The total storage area exceeds 1500 sq. m.
- At all warehouses, serial registration is carried out in electronic form, duplicated on paper








